Ongoing Research Projects
in the Modarelli Group
Organic
Photovoltaics:
Harnessing Solar Energy with Self-Assembly
Photovoltaic devices (PDs) are designed to convert solar energy into electricity. Inorganic PDs are currently the industry standard, but are plagued by high costs of construction. In addition, these materials are rigid and therefore difficult to mold into shapes other than flat panels. Organic PDs are more desirable because of their low cost and the flexible nature of the organic polymers used in their construction that allow for flexible device structures.
In our group we are synthesizing and studying the properties of self-assembled molecules, generally containing porphyrins, metal terpyridines and naphthalene diimides (NDIs), designed to absorb light and convert that can subsequently be converted into energy.
Metal-Ligand Coordination Self-Assemblies
We have recently prepared self-assembled metallo bisterpyridine-NDI polymers (see below) that absorb light and undergo efficient electron-transfer:
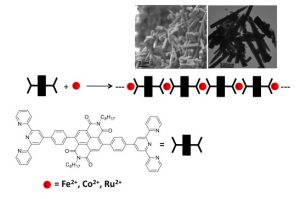
These polymers were shown (cf. Dalton Transactions 2015, 44, 3176-3184.) to self-assemble into nanorods (upper right of graphic, above) composed of several individual polymers.
Hydrogen-Bonding Self-Assemblies
In collaborative work with Ohio State University (Parquette Group) and funded at Akron and OSU by the NSF, we are studying the self-assembly of NDIs using non-covalent H-bonding and pi-stacking interactions to form nanotubes, nanotapes and nanorods. We have subsequently used ultrafast time-resolved laser spectroscopy to study electron and energy transfer in these molecules.
See Tu, S.; Kim, S.H.; Joseph, J.; Modarelli, D.A.; Parquette, J.R. “Self-Assembly of a Donor-Acceptor Nanotube. A Strategy to Create Bicontinuous Arrays” J. Am. Chem. Soc. 2011, 133(47), 19125-19130, highlighted below left,
and
Tu, S.; Kim, S.H.; Joseph, J.; Modarelli, D.A.; Parquette, J.R. “Proton-Coupled Self-Assembly of a Porphyrin Naphthalenediimide (NDI) Dyad” ChemPhysChem 2013, 14, 1609 – 1617, highlighted below right.
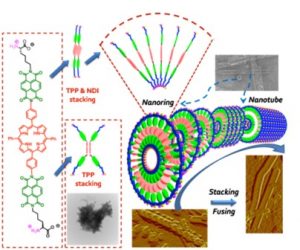

We are also using electrostatic non-covalent interactions (not shown) to assemble NDIs into complex nanotubes.
We use a combination of femtosecond, picosecond and nanosecond transient absorption and time-resolved laser experiments, and time-correlated single photon counting techniques (TCSPC) to study ultrafast electron and energy transfer events. We have a wide variety of laser equipment including an ultrafast femtosecond transient absorption spectrometer (Titan regenetively amplified/multipass laser, and three TOPAS optical parametric amplifiers), a picosecond TCSPC laser system (mode-locked picosecond Nd:YLF laser that pumps a Coherent 700 dye laser, and that is capable of producing 1 ps pulses over a range of wavelengths).
See also:
Shao, H.; Seifert, J.; Romano, N.C.; Gao, M.; Helmus, J.J.; Jaroniec, C.P.; Modarelli, D.A.; Parquette, J.R. “Amphiphilic Self-Assembly of an n-type Nanotube”, Angew. Chem., 2010, 49, 7688 –7691. November Cover Page and a Very Important Paper.
Shao, H.; Nguyen, T.; Romano, N.C.; Modarelli, D.A.; Parquette, J.R. “Self-Assembly of 1-D n-Type Nanostructures based on Naphthalene Diimide-appended Dipeptides” J. Am. Chem. Soc. 2009, 131(45), 16374–16376.
Other Non-Covalent Self-Assemblies
Interactions such as pi-stacking can also lead to formation of highly ordered assemblies. We have recently published work showing porphyrin-C60 conjugates self-assemble into highly columns. Additional work involving pi-stacking in porphyrins and porphyrinoid derivatives is soon-to-be-published.
Wang, C.-L.; Zhang, W.-B.; Yu, X.; Yue, K.; Sun, H.-J.; Hsu, C.-H.; Hsu, C.-S.; Jospeh, J.; Modarelli, D.A.; Cheng, S.Z.D. “Facile Synthesis and Photophysical Properties of Sphere-Square Shape Amphiphiles Based on Porphyrin-[60]Fullerene Conjugates” Chem. Asian J. 2013, 8, 947 – 955.
Synthesis
and Spectroscopy of Unusual Porphyrins
A second major area of interest to us is the synthesis and spectroscopy of porphyrins and porphyrin analogs. In particular, we are interested in preparing unusual porphyrins, understanding their electronic properties (i.e., isomerization/equilibria in the ground and excited states), and excited state dynamics. We also incorporate these molecules as building blocks into larger assemblies we hope will further the development of photovoltaic materials.
Synthesis of these molecules is performed by students interested in synthetic projects.
Photophysical (i.e., steady state and time-resolved fluorescence and pump-probe femtosecond absorption) experiments on N-confused porphyrins, octaphryns, corroles and other unusual porphyrinoid molecules. These experiments are carried out using the various laser spectroscopy tools in our group, and include:
- Femtosecond pump-probe absorption experiments
- Time-correlated single photon counting (TCSPC) experiments
- Nanosecond transient absorption experiments
- Steady state fluorescence experiments
Recent Highlights
 | We recently demonstrated the solvent-dependent isomerization of NCPs: Alemán, E.A.; Joseph, J.; Modarelli, D.A. "Solvent Effects on the Absorption Properties and Tautomerism of N-Confused Tetraphenylporphyrin" J. Org. Chem. 2015, 80, 11031-11038. (Left: Plot showing tautomerization as a function of H-bond acceptor ability of solvent) |
See for example:
Acharya,Rajendra; Paudel, Liladhar; Joseph, Jojo; McCarthy, Claire E.; Dudipala, Venkat R.; Modarelli, Jody M.; Modarelli, David A. “Synthesis of Three Asymmetric N-Confused Porphyrins” J. Org. Chem. 2012, 77(14), 6043–6050.
Alemán, E.A.; Manriquez Rocha, J.; Wongwitwichote, W.; Godinez Mora-Tova, L.A.; Modarelli, D.A. “Spectroscopy of Free-Base N-Confused Tetraphenylporphyrin Radical Cation and Radical Anion” J. Phys. Chem. A 2011, 115, 6456–6471.
Vyas, S.; Hadad, C.; Modarelli, D.A. “TDDFT Calculations of the Structure and Absorption Spectra of Free-Base N-Confused Porphyrin and N-Confused Tetraphenylporphyrin” J. Phys. Chem. A 2008, 112, 6533–6549.
Alemán, E.A.; Rajesh, C.S.; Ziegler, C.S.; Modarelli, D.A. “Ultrafast Spectroscopy of Free Base N-Confused Porphyrins” J. Phys. Chem. A, 2006, 110, 8605-8612.
Wolff, S.A.; Alemán, E.A.; Banerjee, D.; Rinaldi, P.L.; Modarelli, D.A. “One-Step Synthesis and Characterization of Difunctionalized N-Confused Tetraphenylporphyrins” J. Org. Chem. 2004, 69, 4571-4576.
Shaw, J.L.; Wolff, S.A.; Aleman, E.A.; Ziegler, C.J.; Modarelli, D.A. “Synthesis and Spectroscopy of a Series of Substituted N-Confused Tetraphenylporphyrins” J. Org. Chem. 2004, 69, 7423-7427.
Belair, J.P.; Ziegler, C.S.; Rajesh, C.S.; Modarelli, D.A. “Photophysical Characterization of Free-Base N-Confused Tetraphenylporphyrins” J. Phys. Chem. A 2002, 106, 6445-6451.
Chemistry
A fourth area of interest in our group involves the study of molecules using quantum mechanical computational and molecular dynamics programs. We examine the structures and energies of a variety of molecules, from reactive intermediates (see, for example: Modarelli, D.A. J. Org. Chem. 2000, 65, 7277-7283), porphyrins and porphyrinoids (see refs above) and biologically interesting molecules and ions, to polymer monomers. Molecular dynamics is used to examine the conformational space of large macromolecules such as the dendrimers we study in our photosynthetic mimic research. Such studies are important as they allow us to help explain our photophysical data obtained from our laser experiments.